|
|
|
|||||
 |
|||||||
|
|
|||||||
|
|
|||||||
6. Discontinue invisible expiry dates and batch numbers. Regulations to be amended to permit expiry dates and batch number details to be printed in smudge-proof black on a white background
|
|||||||
7. EXPIRY DATES + BATCH NUMBERS + PRODUCT NAME + STRENGTH + BAR-CODE to be including on the END of dispensary packs. This allows simple storage, stock control, and stock rotation.
|
|||||||
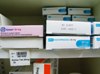
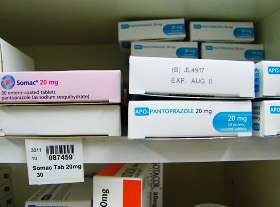
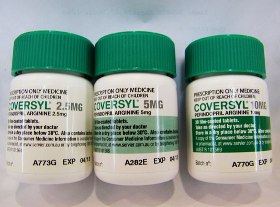
8. Similar packaging for different strengths of the same drug by the same manufacturer can lead to potential selection errors. AN ACCIDENT WAITING TO HAPPEN!
|
|||||||
9. DD's with bad packaging e.g. Codeine phos. tablets labelled on only 1 facing of their pack
|
|||||||
| 10. Include a list of all the generic bio-equivalent brand names to AVOID DOUBLE-DOSING of the same medication to the unsuspecting patient. Could either be on the packaging, or as an insert | |||||||
| 11. Cautionary / advisory information to be printed clearly on the label for the patient. Sometimes the pertinent information is unable to fit onto one dispensary label, causing the second label to obliterate necessary manufacturer's product details. | |||||||
| 12. Providing manufacturer's free-call enquiry contact details e.g. a/ 1800 telephone and b/ website links | |||||||
| 13. hot-link to Poisons Info hotline website:http://www.chw.edu.au/poisons/index.htm and Poisons Info Hotline Tel No = 13 11 26 |
|||||||
| 14. ‘www.mothersafe.org.au’ 1800 hotline and website www.mothersafe.org.au for pregnant or breastfeeding mums concerned about the safety of their relevant medication. | |||||||
15. Ensuring various BRAND-NAMES of drugs will NOT CAUSE CONFUSION with PRESCRIBERS AND/ OR DISPENSERS Generic name confusion issues: |
|||||||
| 16. Highlight printing of brand-name, generic name AND strength, relative to manufacturer's details | |||||||
 |
|||||||
|
|
|
|
|
|
|
|
|